
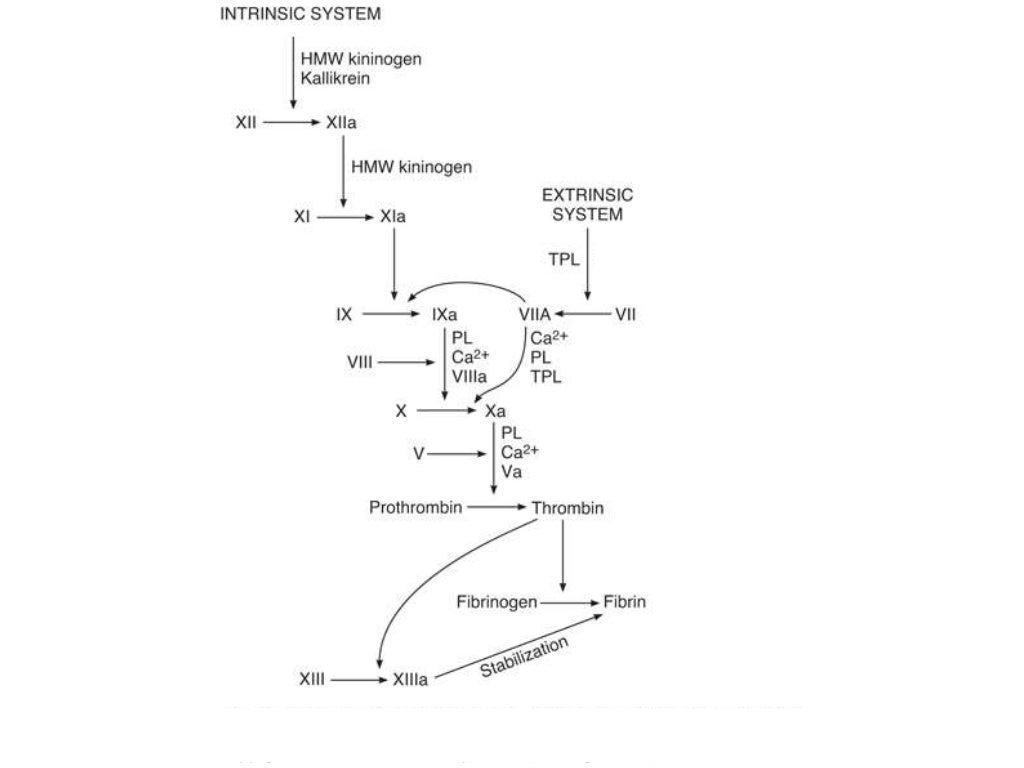
In contrast to warfarin, the NOACs directly inhibit a single clotting enzyme dabigatran inhibits thrombin, whereas rivaroxaban, apixaban, and edoxaban inhibit factor Xa. Sites of action of warfarin and the non-vitamin K oral anticoagulants. Because of its indirect mechanism of action, the onset and offset of action of warfarin are delayed for several days, a phenomenon that often necessitates bridging with a rapidly acting parenteral anticoagulant when initiating warfarin therapy, and complicates periprocedural management. This results in attenuated thrombin generation regardless of whether clotting is triggered via the extrinsic, intrinsic, or common pathway of coagulation. Without reduced vitamin K as a cofactor for hepatic γ-carboxylase, functional levels of the vitamin K–dependent clotting proteins, factors II, VII, IX, and X decrease. Warfarin inhibits vitamin K epoxide reductase, thereby attenuating the reduction of oxidized vitamin K in the liver. Although not approved in the United States for this indication, rivaroxaban is licensed in Europe for the prevention of recurrent ischemia in stabilized patients with acute coronary syndrome (ACS). In the United States, rivaroxaban and apixaban are licensed for prevention of venous thromboembolism (VTE) after elective hip or knee replacement surgery and dabigatran, rivaroxaban, apixaban, and edoxaban are approved for treatment of VTE and for stroke prevention in patients with atrial fibrillation (AF). This is an important advantage because bleeding into the brain is the most feared complication of anticoagulation therapy. Moreover, as a class, the NOACs are associated with significantly less intracranial bleeding than warfarin. Designed to overcome the limitations of warfarin, the NOACs have revolutionized oral anticoagulation because they are at least as effective as warfarin, but are more convenient to administer because the NOACs can be given in fixed doses without routine coagulation monitoring. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa.
Inhibits the activity of the Tissue Factor Pathway Inhibitor (TFPI), increasing tissue factor-initiated thrombin generation.Binds and sequesters factor Xa inhibitors.



 0 kommentar(er)
0 kommentar(er)
